In this study, the in silico model examined 14 bioactive compounds from Carica papaya leaves (Table 1) alongside two reference drugs against five protein targets. Results indicate that carpaine, myricetin 3-rhamnoside, orientin 7-O-rhamnoside, and quercetin demonstrated the strongest binding scores. These compounds, therefore, hold potential as inhibitors of alpha-amylase, beta-glucosidase, DPP-4, PPARG, SGLT-2, and SUR-1 (Table 2), offering diverse pathways for managing diabetes. This aligns with findings by Kong, et al.12 and suggests the potential of Carica papaya-based diabetes treatments. In another research, bioactive compounds in natural compounds, such as quercetin, have been found to inhibit key enzymes involved in carbohydrate digestion, including alpha-amylase and beta-glucosidase13. By inhibiting these enzymes, AECP reduces the rate of carbohydrate breakdown and glucose absorption, thereby lowering blood glucose levels. These findings suggest that developing Carica papaya-based treatments could help manage diabetes.
Molecular investigations were undertaken on the inhibitory activities of the relative compounds in the Carica papaya leaves (Table 2). Two reference medicines (acarbose and metformin) were used to compare the docking score with the compounds in Carica papaya leaves. Four comparable compounds exhibited a good docking score compared to the two reference medicines utilized against various macromolecular targets. The four compounds with good docking scores were carpaine, myricetin 3-rhamnoside, orientin 7-O-rhamnoside and quercetin. These results revealed the following components might be the bioactive compounds contained in Carica papaya leaves that can potentially regulate OS complications like diabetes.
The data in Table 3 reveal the pharmacokinetic properties and bioavailability of various metabolites, examining key characteristics including GI absorption potential, ability to cross the BBB, P-gp substrate status, bioavailability score, and skin permeation (Log Kp). Differences in GI absorption values are observed among the metabolites, with myricetin 3-rhamnoside and orientin 7-O-rhamnoside showing low absorption potential, while quercetin, caffeic acid, carpaine, and protocatechuic acid exhibit high GI absorption values, indicating more effective absorption after oral intake. Five of the metabolites, including equisetin and ferulic acid could penetrate the BBB, implying potential activity on the central nervous system. Some metabolites are identified as P-gp substrates, such as equisetin and orientin 7-O-rhamnoside, influencing absorption processes in the body. Skin permeation values suggest that myricetin 3-rhamnoside, prunasin, and orientin 7-O-rhamnoside have poor skin penetration, while carpaine, Hexadecanamide and 9-octadecenamide show better absorption through the skin. Water solubility varies, with compounds like caffeic acid and protocatechuic acid being very soluble, while carpaine is poorly soluble. High TPSA in some compounds may limit permeability. Several compounds inhibit CYP enzymes, notably quercetin, equisetin, and 9-octadecenamide, indicating potential for drug–drug interactions. The bioavailability score indicates that most metabolites possess moderate scores, with compounds including ferulic acid, equisetin, quercetin, caffeic acid, and tenuazonic acid displaying high scores, suggesting their more effective use within the body. Overall, these findings reveal significant differences in the pharmacokinetic profiles of the metabolites, which may have important implications for their potential therapeutic effects, particularly for those with high GI absorption and bioavailability scores that warrant further investigation.
The toxicity prediction result for the fourteen compounds is presented in Table 4. Most compounds demonstrated low potential for hERG channel blocking, with values generally below 0.7, indicating a relatively low risk of cardiac arrhythmia. However, 9-octadecenamide (0.68) and carpaine (0.57) approached higher values, suggesting that their cardiotoxic potential should be monitored. In contrast, compounds like ferulic acid, caffeic acid, tenuazonic acid, and protocatechuic acid had minimal hERG blocking predictions (0.01), indicating a safer cardiac profile. Regarding clinical toxicity, most of the compounds showed low predictions, particularly 2-methoxy-4-vinylphenol (0.0021), and tenuazonic acid and hexadecanamide (0.01), suggesting a favorable safety profile in clinical settings. However, 11-hydroperoxy-12,13-epoxy-9-octadecenoic acid (0.35) was notably higher, implying a need for caution. Predictions for DILI were particularly high for myricetin 3-rhamnoside, quercetin (both 0.93), and orientin 7-O-rhamnoside (0.88), highlighting a potentially significant hepatotoxic risk. This suggests their potential toxicity at high doses. On the other hand, hexadecanamide (0.12) and 9-octadecenamide (0.11) had lower DILI predictions, indicating better liver safety. Most compounds exhibited low carcinogenicity predictions, with quercetin, ferulic acid, and 2-methoxy-4-vinylphenol all at or below 0.03. However, 9-octadecenamide (0.70), hexadecanamide (0.64), and tenuazonic acid (0.43) had elevated values, suggesting a possible carcinogenic concern. In acute toxicity, measured as LD50 in log(1/mol/kg), higher values reflect lower toxicity. Equisetin (3.81), myricetin 3-rhamnoside (3.38), and orientin 7-O-rhamnoside (3.14) exhibited the highest LD50 values, suggesting lower acute toxicity. Lastly, for skin reaction, compounds like 9-octadecenamide (0.92), quercetin (0.89), and 11-hydroperoxy-12,13-epoxy-9-octadecenoic acid (0.84) had high predictions, implying potential for dermal irritation or allergic response.
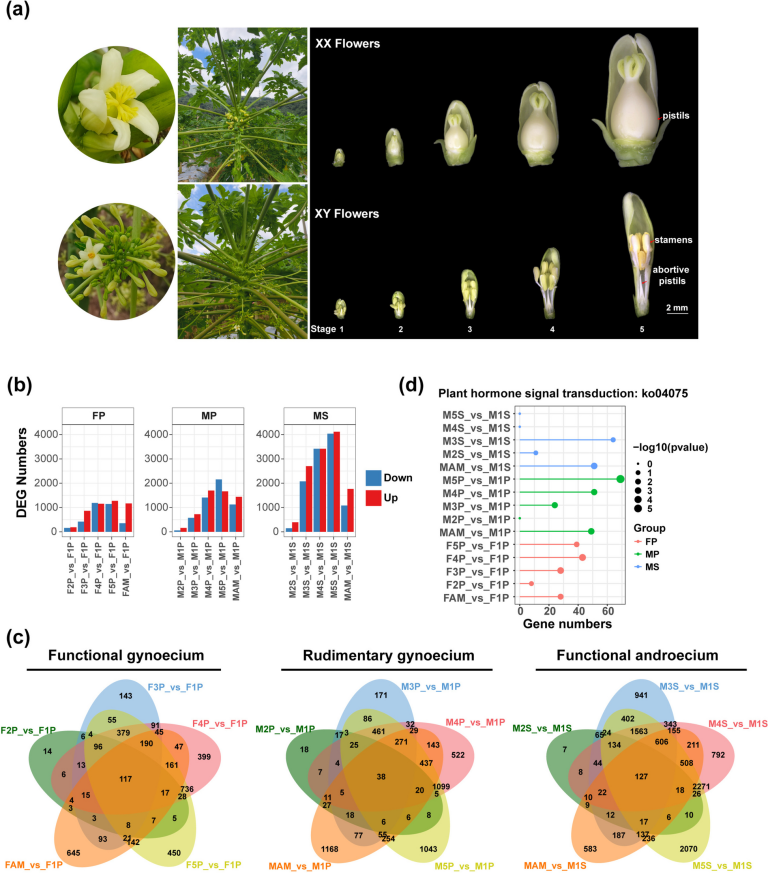
In this study, the endocrine-disrupting potential of fourteen selected metabolites was evaluated using the Endocrine Disruptome platform, and the results are presented in Figs. 1, 2, 3 and 4. Each compound was assessed for its binding probability to 15 nuclear receptors, including AR, ER α/β, GR, MR, PR, PPAR α/β/γ, TR α/β, RXR α, and LXR α/β. The predicted binding scores were classified based on interaction likelihood as high (red), moderate (orange), or low (green).
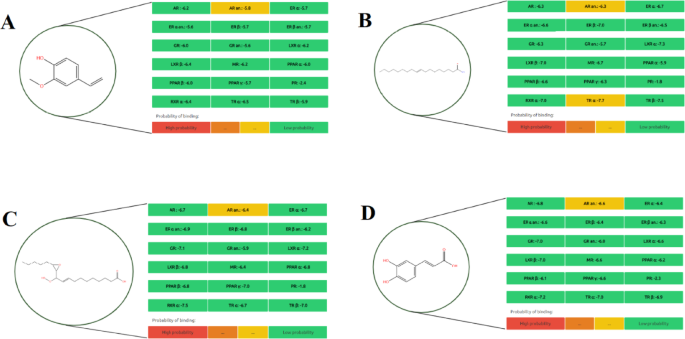
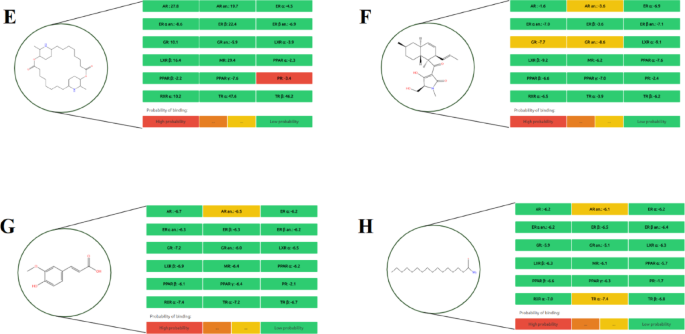
Among all tested compounds, quercetin (Fig. 4L) demonstrated the highest binding potential across multiple receptors, particularly AR, GR, MR, PPAR γ, and TR α, indicating a broad spectrum of endocrine-disrupting capacity. Similarly, carpaine (Fig. 2E) exhibited high-affinity binding specifically to the PR receptor, warranting consideration as a potential endocrine-active compound. Orientin 7-O-rhamnoside (Fig. 3İ) showed a moderate binding probability to the PR receptor, suggesting selective interaction potential.
Other metabolites such as ferulic acid (Fig. 2G), protocatechuic acid (Fig. 3J), prunasin (Fig. 3K), and tenuazonic acid (Fig. 4M) demonstrated moderate binding only to the AR α receptor, while exhibiting low affinity to other targets, indicating limited endocrine-disrupting potential. In contrast, compounds including 2-methoxy-4-vinylphenol (Fig. 1A), 9-octadecenamide (Fig. 1B), 11-hydroperoxy-12,13-epoxy-9-octadecenoic acid (Fig. 1C), hexadecanamide (Fig. 2H), equisetin (Fig. 2F), and myricetin 3’-rhamnoside (Fig. 3I) displayed low binding across all nuclear receptors, suggesting minimal endocrine interference.
Overall, AR α and PR emerged as the most frequently affected receptors, with several metabolites showing moderate to high affinity toward them. These findings highlight receptor-specific binding tendencies of phytochemical metabolites and suggest that a subset of these compounds may exhibit endocrine-disrupting behavior, especially through interaction with androgenic and progestogenic pathways.
The predicted endocrine disruption potential of four key metabolites: (A) 2-Methoxy-4-vinylphenol, (B) 9-Octadecenamide, (C) 11-Hydroperoxy-12,13-epoxy-9-octadecenoic acid, and (D) Caffeic Acid.
The predicted endocrine disruption potential of four key metabolites: (E) Carpaine, (F) Equisetin, (G) Ferulic acid, and (H) Hexadecanamide.
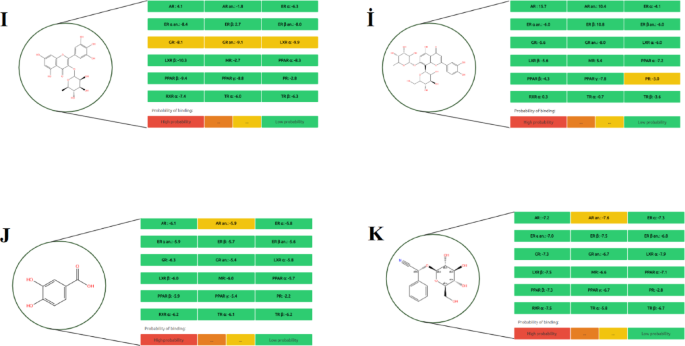
The predicted endocrine disruption potential of four key metabolites: (I) Myricetin 3’-rhamnoside, (İ) Orientin 7-O-rhamnoside, (J) Protocatechuic acid, and (K) Prunasin.
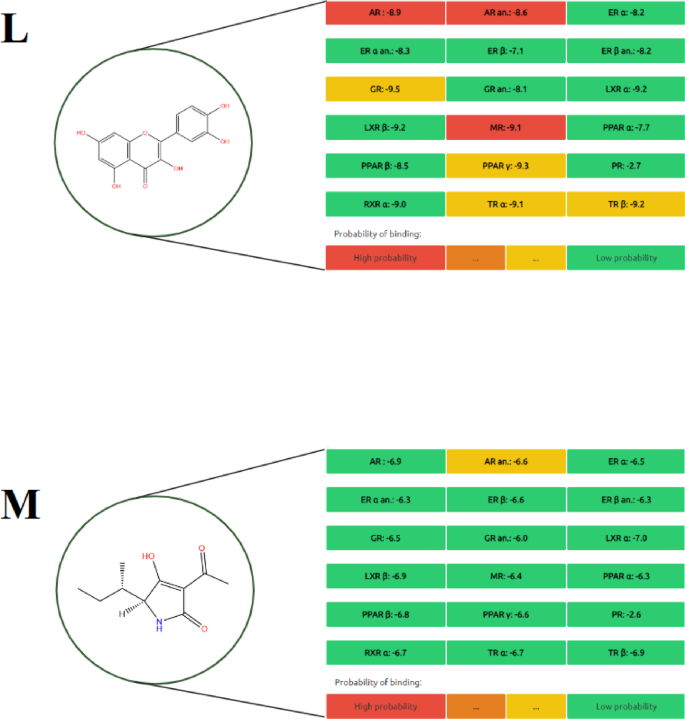
The predicted endocrine disruption potential of four key metabolites: (L) Quercetin, (M) Tenuazonic acid.
Oxidative stress results from an excess of ROS, thereby, causing harmful oxidation and inflammation. These processes have been linked to various health conditions, including diabetes, Alzheimer’s disease, rheumatoid arthritis, cancers, cardiovascular diseases, cataracts, and even cosmetic issues like wrinkles12.
The fruit fly, Drosophila melanogaster, is a valuable model for investigating human health due to the significant homology between its genome and that of humans. Its short lifespan, ease of maintenance, and well-understood genetics make it a powerful tool for studying diseases like cancer and developmental studies14. One of the major parameters used to assess the effect of plants and plant products in D. melanogaster is the survival rate15. It was noted that low doses of the AECP (50 and 100 mg/kg diet) improved the survival rate of the flies, which suggests that caution should be taken with the consumption of AECP at high doses (Fig. 5). The observed safe doses (50 and 100 mg/kg diet) were, therefore, used to investigate the protective effect against high-sucrose-induced hyperglycemia and oxidative stress in fruit flies.
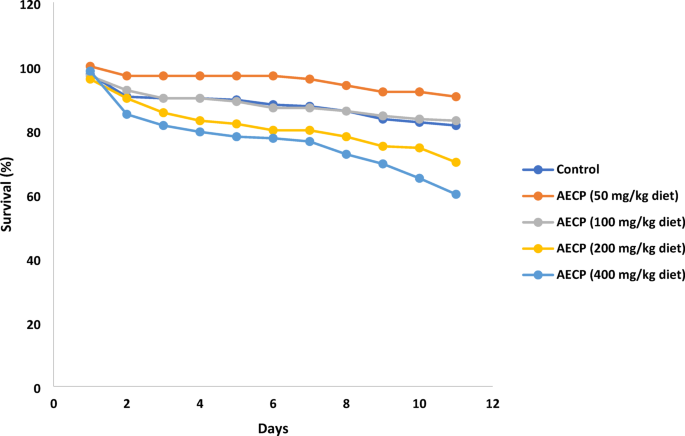
Effect of aqueous extract of Carica papaya leaf on 12-day survival rate in adult D. melanogaster.
Previous studies indicate that sucrose consumption elevates glucose levels, and excess sucrose consumption can trigger OS and its complications16,17. The present study explores the potential of AECP as a countermeasure. A significant (p < 0.05) increase in the level of glucose was noted in flies fed with sucrose alone and in sucrose-fed flies treated with 50 and 100 mg/kg AECP when compared with the control (Fig. 6). Furthermore, a significant (p < 0.05) reduction in glucose level was observed in the sucrose-fed diet fortified with 50 mg/kg AECP compared to the sucrose-only treated group. Results indicate that AECP reversed the glucose elevation caused by a high sucrose diet in the flies. This finding supports the result of the in silico analysis which suggest that the hit compounds (bioactive compounds) in the Carica papaya may interact with the diabetic protein targets to reduce the excess glucose level.
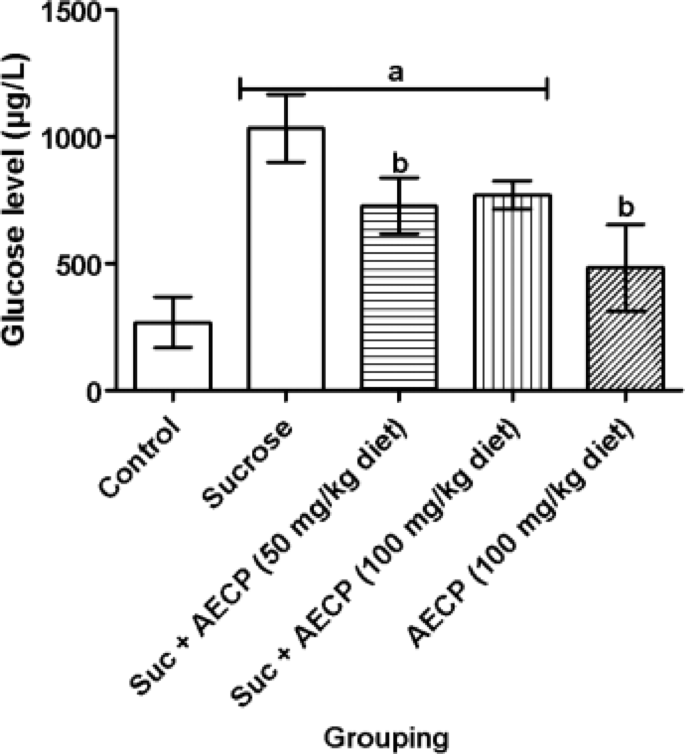
Effect of aqueous extract of Carica papaya leaf on glucose level in sucrose-induced oxidative stress in flies. Data are presented as Mean ± SD of 40 flies/vial (n = 5). ap < 0.05 when compared with the control, bp < 0.05 when compared with sucrose only. AECP: Aqueous extract of Carica papaya; Suc: Sucrose.
The antioxidant potential of Carica papaya has been documented in various studies. The high antioxidant activity of Carica papaya extracts can be attributed to bioactive compounds such as alkaloids, flavonoids, saponins, glycosides, phytosterol, flavonoids, tannins terpenoids, and vitamins5. These compounds neutralize free radicals and boost the body’s antioxidant defense system. The role of catalase and GST in maintaining cellular health shows the importance of these enzymes in mitigating the effects of OS. Catalase, by breaking down hydrogen peroxide, prevents the formation of hydroxyl radicals, one of the most reactive and damaging types of free radicals18. Glutathione-s-transferase, through its detoxification role, helps eliminate reactive intermediates that could otherwise contribute to cellular damage and dysfunction as seen in this study.
In this study, the level of total thiols was significantly (p < 0.05) reduced in flies fed with a high-sucrose diet only and groups treated with a sucrose diet fortified with 50 and 100 mg/kg AECP when compared to the control group (Fig. 7). However, a significant (p < 0.05) increase in the level of total thiols in flies treated with 100 mg/kg AECP-only was observed when compared with sucrose-only fed flies. Also, a significant (p < 0.05) decrease in catalase activity was observed in sucrose-only treated flies when compared to the control (Fig. 8). The catalase activity was significantly (p < 0.05) increased in flies treated with both doses of AECP when compared to the group treated with sucrose only. The GST activity was significantly (p < 0.05) decreased in the group treated with sucrose only when compared to the control group and the group treated with 50 mg/kg diet AECP (Fig. 9). The GST activity was also significantly increased (p < 0.05) in the groups treated with AECP (100 mg/kg) when compared to the group treated with sucrose only. This could be associated with the antioxidant activity of the AECP. In addition, the binding score of carpaine as a potential compound, could enhance catalase activity as observed in the in silico analysis.
It has been reported that fruit extract of papaya increased the activities of antioxidant enzymes in biological systems, and ameliorated lipid peroxidation5supporting the notion that Carica papaya can enhance the body’s ability to manage OS. This aligns with the findings of this study, where the antioxidant effects of AECP restored enzyme activities and potentially improved overall cellular health and resilience against OS. This study adds to the growing body of evidence supporting the health benefits of Carica papaya, particularly its role in enhancing antioxidant defenses. The restoration of total thiols level, catalase and GST activities in the treatment groups showed the potential of Carica papaya as a natural remedy for managing OS and its associated complications. These findings are particularly relevant in dietary management and the potential use of Carica papaya in functional foods and nutraceuticals to reduce OS and improve overall health.
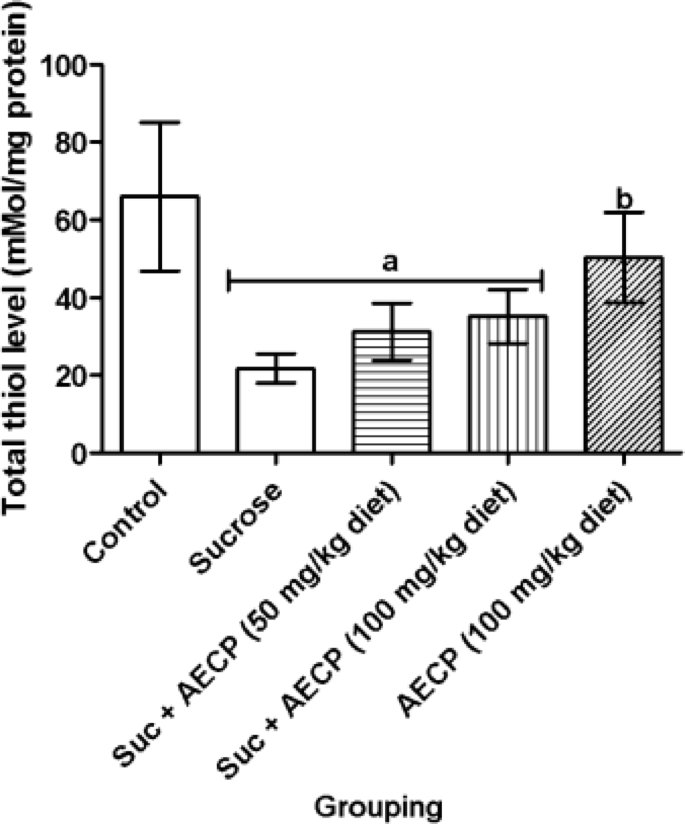
Effect of aqueous extract of Carica papaya leaf on the level of total thiol in sucrose-induced oxidative stress in flies. Data are presented as Mean ± SD of 40 flies/vial (n = 5). ap < 0.05 when compared with the control, bp < 0.05 when compared with sucrose only. AECP: Aqueous Extract of Carica papaya; Suc: Sucrose.
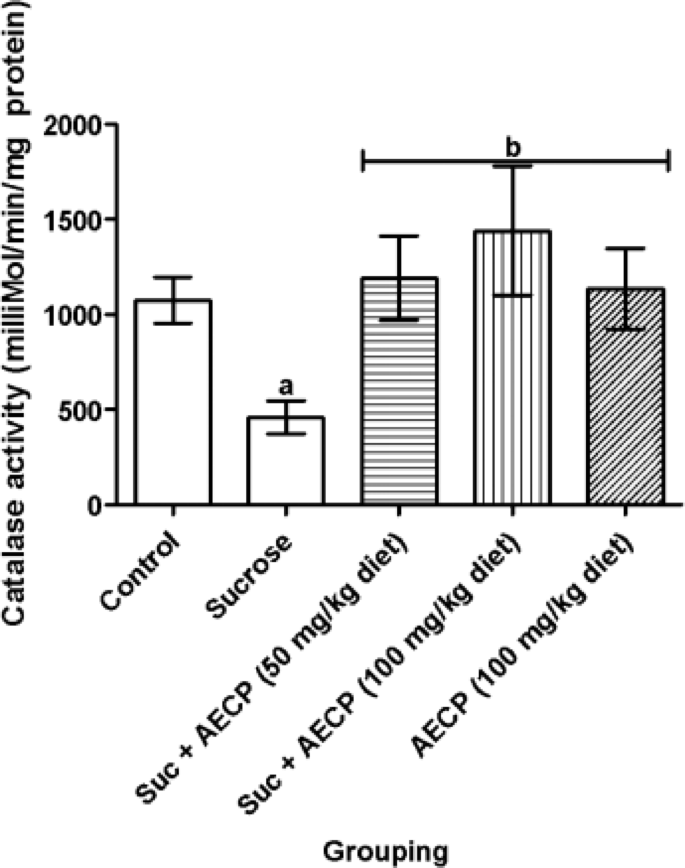
Effect of aqueous extract of Carica papaya leaf on the activity of catalase in sucrose-induced oxidative stress in flies. Data are presented as Mean ± SD of 40 flies/vial (n = 5). ap < 0.05 when compared with the control, bp < 0.05 when compared with sucrose only. AECP: Aqueous extract of Carica papaya; Suc: Sucrose.
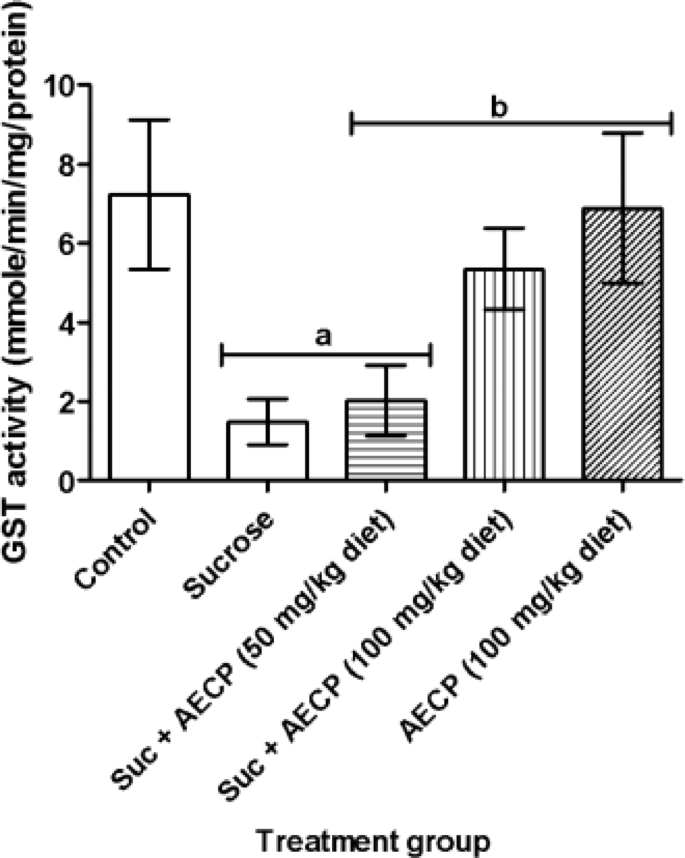
Effect of aqueous extract of Carica papaya on the glutathione-s-transferase activity in sucrose-induced oxidative stress in flies. Data are presented as Mean ± SD of 40 flies/vial (n = 5). ap < 0.05 when compared with the control, bp < 0.05 when compared with sucrose only. AECP: Aqueous Extract of Carica papaya; Suc: Sucrose.
Elevated level of glucose (hyperglycemia) is associated with elevated production of NO through elevated expression of inducible NO synthase (iNOS) and endothelial NO synthase (eNOS) gene and protein levels19. In this study, a significant (p < 0.05) increase in the level of NO was noted in flies exposed to the sucrose diet only when compared to the control (Fig. 10). However, a significant (p < 0.05) reduction was noted in the sucrose diet-fed flies treated with both doses of AECP compared to the sucrose diet only. This suggests the ability of AECP to inhibit the production of NO.
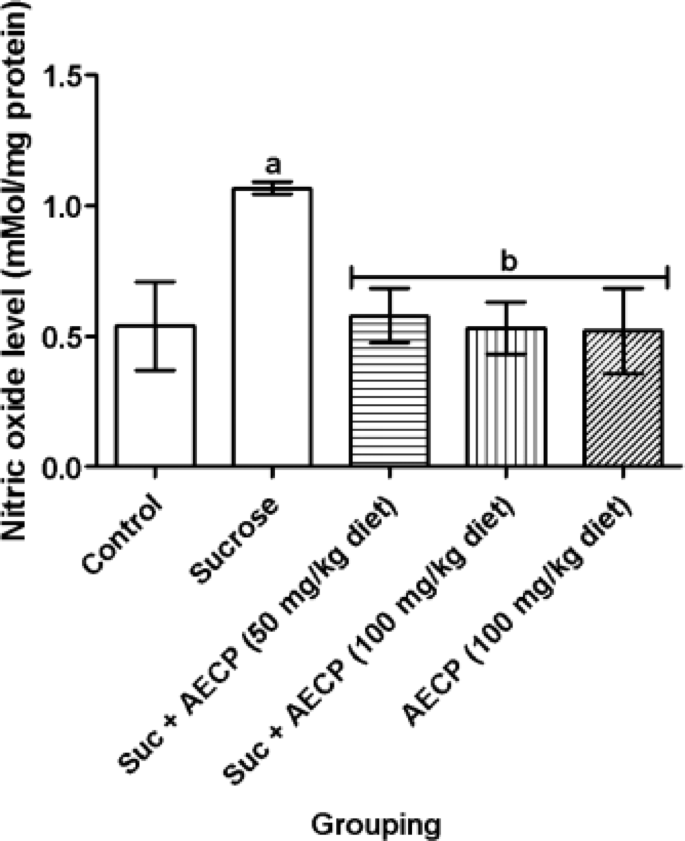
Effect of aqueous extract of Carica papaya leaf on the level of nitric oxide in sucrose-induced oxidative stress in flies. Data are presented as Mean ± SD of 40 flies/vial (n = 5). ap < 0.05 when compared with the control, bp < 0.05 when compared with sucrose only. AECP: Aqueous extract of Carica papaya; Suc: Sucrose.